Abstract:
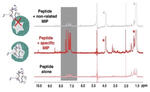
Molecularly imprinted polymers (MIPs) are tailor-made synthetic antibodies possessing specific binding cavities designed for a target molecule. Currently, MIPs for protein targets are synthesized by imprinting a short surface-exposed fragment of the protein, called epitope or antigenic determinant. However, finding the epitope par excellence that will yield a peptide ‘synthetic antibody’ cross-reacting exclusively with the protein from which it is derived, is not easy. We propose a computer-based rational approach to unambiguously identify the ‘best’ epitope candidate. Then, using Saturation Transfer Difference (STD) and WaterLOGSY NMR spectroscopies, we prove the existence of specific binding sites created by the imprinting of this peptide epitope in the MIP nanogel. The optimized MIP nanogel could bind the epitope and cognate protein with a high affinity and selectivity. The study was performed on Hepatitis A Virus Cell Receptor-1 protein, also known as KIM-1 and TIM-1, for its ubiquitous implication in numerous pathologies.